Number Of Electrons In Calcium 40
Calcium oxide - CaO Ca: 20 electrons Oxygen: 8 electrons Total: 28. Depends on what you mean by number of electrons. Calcium has 20 electrons and oxygen has 8 electrons for a total of 28 electrons. Number of valence electrons in Calcium. August 27, 2014, charm, Leave a comment. How many valence electrons are there in Calcium? Do you like chemistry? Keep in mind the fact that Calcium can be found in the 4th energy level of the periodic table, and in the 2nd group (column).
Atomic number of Ca is 20.The electronic configuration is; 2, 8, 8, 2. Thus, the number of electrons in the penultimate shell(3rd) are 8. Answer verified by Toppr Upvote (0). The calcium atom transfers electrons to chlorine atoms, leading to the formation of chemical bonds. Calcium chloride contains - bonds. Copper atoms are used to produce pennies. The copper atoms in pennies share many electrons. The number of valence electrons an atom has. How many valence electrons does antimony have?
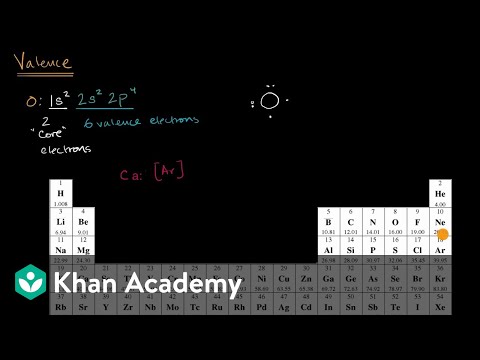
Calcium has 2 valence electrons. Calcium has 20 electrons so they are arranged 2. 2 Using orbital notation the electron structure is: 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2) You can see that there are 2 electrons in the outer energy level and these are the valence electrons.
Element Calcium - Ca
Comprehensive data on the chemical element Calcium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Calcium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Calcium Menu
- Calcium Page One
- Calcium Page Two
- Calcium Page Three
Overview of Calcium
- Atomic Number: 20
- Group: 2
- Period: 4
- Series: Alkali Earth Metals
Calcium's Name in Other Languages
- Latin: Calcium
- Czech: Vápník
- Croatian: Kalcij
- French: Calcium
- German: Kalzium - r
- Italian: Calcio
- Norwegian: Kalsium
- Portuguese: Cálcio
- Russian: Кальций
- Spanish: Calcio
- Swedish: Kalcium
Atomic Structure of Calcium

- Atomic Radius: 2.23Å
- Atomic Volume: 29.9cm3/mol
- Covalent Radius: 1.74Å
- Cross Section (Thermal Neutron Capture)σa/barns: 0.43
- Crystal Structure: Cubic face centered
- Electron Configuration:
- 1s2 2s2p6 3s2p6 4s2
- Electrons per Energy Level: 2,8,8,2
- Shell Model
- Shell Model
- Ionic Radius: 0.99Å
- Filling Orbital: 4s2
- Number of Electrons (with no charge): 20
- Number of Neutrons (most common/stable nuclide): 20
- Number of Protons: 20
- Oxidation States: 2
- Valence Electrons: 4s2
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Calcium
- Electrochemical Equivalent: 0.7477g/amp-hr
- Electron Work Function: 2.87eV
- Electronegativity: 1 (Pauling); 1.04 (Allrod Rochow)
- Heat of Fusion: 8.54kJ/mol
- Incompatibilities:
- water, oxidizers, acids, air, chlorine, chlorine tri-fluoride, fluorine, oxygen, silicon, sulfur
- Ionization Potential
- First: 6.113
- Second: 11.871
- Third: 50.908
- Valence Electron Potential (-eV): 29
Physical Properties of Calcium
- Atomic Mass Average: 40.078
- Boiling Point: 1757K 1484°C 2703°F
- Coefficient of lineal thermal expansion/K-1: 22E-6
- Conductivity
- Electrical: 0.298 106/cm Ω
Thermal: 2.01 W/cmK
- Electrical: 0.298 106/cm Ω
- Density: 1.55g/cc @ 300K
- Description:
- silvery, soft metal, tarnishes to grayish white after exposure to air.
- Elastic Modulus:
- Bulk: 17/GPa
- Rigidity: 7.4/GPa
- Youngs: 20/GPa
- Enthalpy of Atomization: 184 kJ/mole @ 25°C
- Enthalpy of Fusion: 8.54 kJ/mole
- Enthalpy of Vaporization: 150 kJ/mole
- Flammablity Class: Flammable solid
- Freezing Point:see melting point
- Hardness Scale
- Brinell: 167 MN m-2
- Mohs: 1.75
- Heat of Vaporization: 153.6kJ/mol
- Melting Point: 1112K 839°C 1542°F
- Molar Volume: 26.02 cm3/mole
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 0.632J/gK
- Vapor Pressure = 254Pa@839°C
Regulatory / Health
- CAS Number
- 7440-70-2
- RTECS: EV8040000
- NFPA 704
- Health: 1
- Fire: 1
- Reactivity: 2
- Special Hazard:
- OSHAPermissible Exposure Limit (PEL)
- No limits set by OSHA
- OSHA PEL Vacated 1989
- No limits set by OSHA
- NIOSHRecommended Exposure Limit (REL)
- No limits set by NIOSH
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 60.5
- Bone/p.p.m: 170000
- Liver/p.p.m: 100-360
- Muscle/p.p.m: 140-700
- Daily Dietary Intake: 600-1400 mg
- Total Mass In Avg. 70kg human: 1 kg
Who / Where / When / How
- Discoverer: Sir Humphrey Davy
- Discovery Location: London England
- Discovery Year: 1808
- Name Origin:
- Latin: calx, calcis (lime).
- Abundance of Calcium:
- Earth's Crust/p.p.m.: 41000
- Seawater/p.p.m.: 390
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): 2240000
- Sources of Calcium:
- Obtained from minerals like chalk, limestone & marble. Very abundant. Makes up 3.5% of crust. Occurs only in compounds. World production in 2000 was around 112,000,000 tons (CaO). Calcium is mined almost everywhere.
- Uses of Calcium:
- Used for dehydrating oils, decarburization and desulfurization of iron and its alloys, getter in vacuum tubes. Also used as an alloying agent for aluminum, copper and lead, a reducing agent for beryllium and used in fertilizer, concrete & plaster of paris. Calcium is an essential component shells, bones, teeth and plant structures.
- Additional Notes:
- Calcium was prepared as lime by the Romans under the name calyx in the 1st century A.D., but the metal was not discovered until 1808. Berzelius and Pontin prepared calcium amalgam by electrolizing lime in mercury. Davy was then successful in isolating the impure metal. Why did it take so long? Calcium is the fifth most abundant metalic element in the earth's crust, but is never found in the elemental form because it is so reactive. It is found in limestone (CaCO3) gypsum (CaSO4. 2H2O) and fluorite (CaF2). Pure calcium is a shiny soft metal that will react violently with water to produce hydrogen.
Calcium Menu
- Calcium Page One
- Calcium Page Two
- Calcium Page Three
Calcium Number Of Protons
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page. Clean my iphone for mac.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:

Kenneth Barbalace. Periodic Table of Elements - Calcium - Ca. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/Ca.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Ca.html'>echo Periodic Table of Elements: Calcium - Ca (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Calcium - Ca is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.
Number Of Electrons In Calcium Ion
June 2018 Chemistry Regents #1-5
Highlight to reveal answers and explanations
Questions | Answer | Links | Explanations |
| 1 Which statement describes the charge and location of an electron in an atom? (1) An electron has a positive charge and is located outside the nucleus. (2) An electron has a positive charge and is located in the nucleus. (3) An electron has a negative charge and is located outside the nucleus. (4) An electron has a negative charge and is located in the nucleus. | 3 | electrons..negative..outside nucleus protons..positive..in nucleus | |
| 2 Which statement explains why a xenon atom is electrically neutral? (1) The atom has fewer neutrons than electrons. (2) The atom has more protons than electrons. (3) The atom has the same number of neutrons and electrons. (4) The atom has the same number of protons and electrons. | 4 | neutral..postive equals negative protons equal electrons | |
| 3 If two atoms are isotopes of the same element, the atoms must have (1) the same number of protons and the same number of neutrons (2) the same number of protons and a different number of neutrons (3) a different number of protons and the same number of neutrons (4) a different number of protons and a different number of neutrons | 2 | isotopes..same number protons different number of neutrons | |
| 4 Which electrons in a calcium atom in the ground state have the greatest effect on the chemical properties of calcium? (1) the two electrons in the first shell (2) the two electrons in the fourth shell (3) the eight electrons in the second shell (4) the eight electrons in the third shell | 2 | valence electrons are the ones that determine chemical properties highest energy level electrons | |
| 5 The weighted average of the atomic masses of the naturally occurring isotopes of an element is the Build for mac on linux. (1) atomic mass of the element (2) atomic number of the element (3) mass number of each isotope (4) formula mass of each isotope | 1 | definition of atomic mass |